
.jpg)
.jpg)
Rapid, Lab-Accurate Diagnostics.
From Clinic to Point-of-Care
Diagnostic Confidence at the Point-of-Care
Critical healthcare decisions require immediate data. Our point-of-care solutions empower clinicians with the quantitative results they need, when they need them.
Looking to Create Custom Test Panels?
Partnership Enquiry
Explore Our Core Technologies

μFluidix
High-sensitivity multiplexed, plug-and-play platform capable of matching clinical lab gold standards.

QuantALFA
Qualitative & Quantitative lateral flow tests matching the clinical lab gold standards.

USelect
Magnetic bead based sperm separation. Superior selection for improved ART success rates.
Our Products Include:
| Platform | Available Panels | |
|---|---|---|
| μFluidix | Fertility, Thyroid, Respiratory | View Details » |
| QuantALFA | Cystatin C, CRP, hs-CRP | View Details » |
| USelect | Magnetic Bead-Based Sperm Separation | View Details » |
Ready to bring lab-quality diagnostics to your practice?

μFluidix: Multiplexed microfluidics cartridge analyzer
High-sensitivity, quantitative results at the point of care.
The μFluidix Reader

Rapid Results
Results in ~35 minutes
Multiplexed Testing
Up to 5 analytes simultaneously
Fully Automated
No manual intervention required
Intuitive Interface
Minimal steps, easy operation
The μFluidix Cartridge
Single Use
Disposable cartridge design
Low Sample Volume
Only 50 µL required
Ready to Use
Pre-loaded with room temperature stable reagents
Smart Analyte Grouping
Targeted panels for the right combination of markers.

Simple Workflow
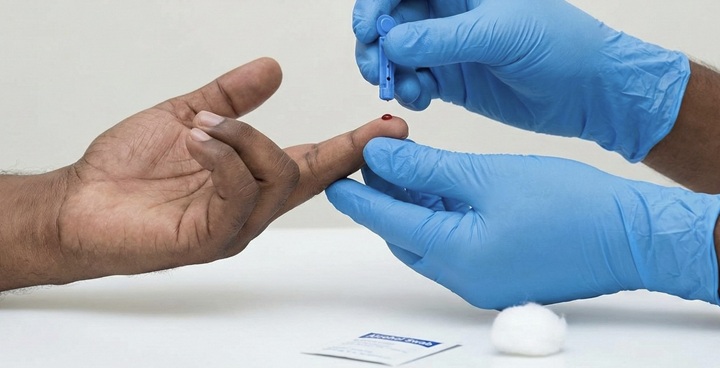
Obtain sample
Prick the fingertip with a sterile lancet to collect the blood sample.
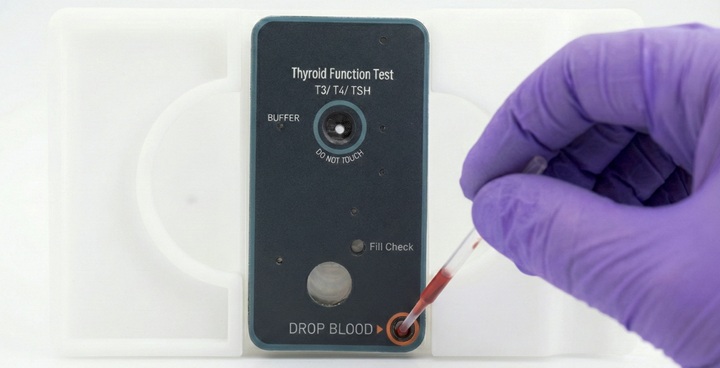
Add sample
Add the patient sample to the test cartridge.
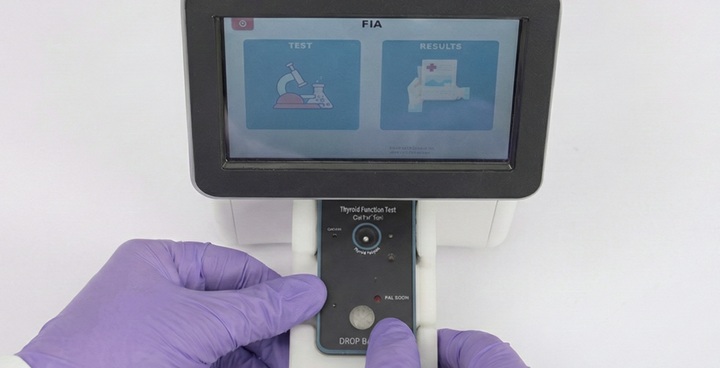
Load cartridge
Place cartridge into the analyzer.
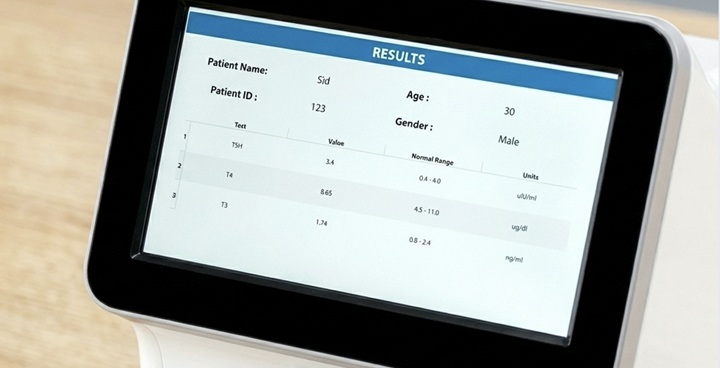
View Results
Run the test and view results within 35min.
See How It Works
Next Generation Diagnostics

Porous Hydrogel Sensors
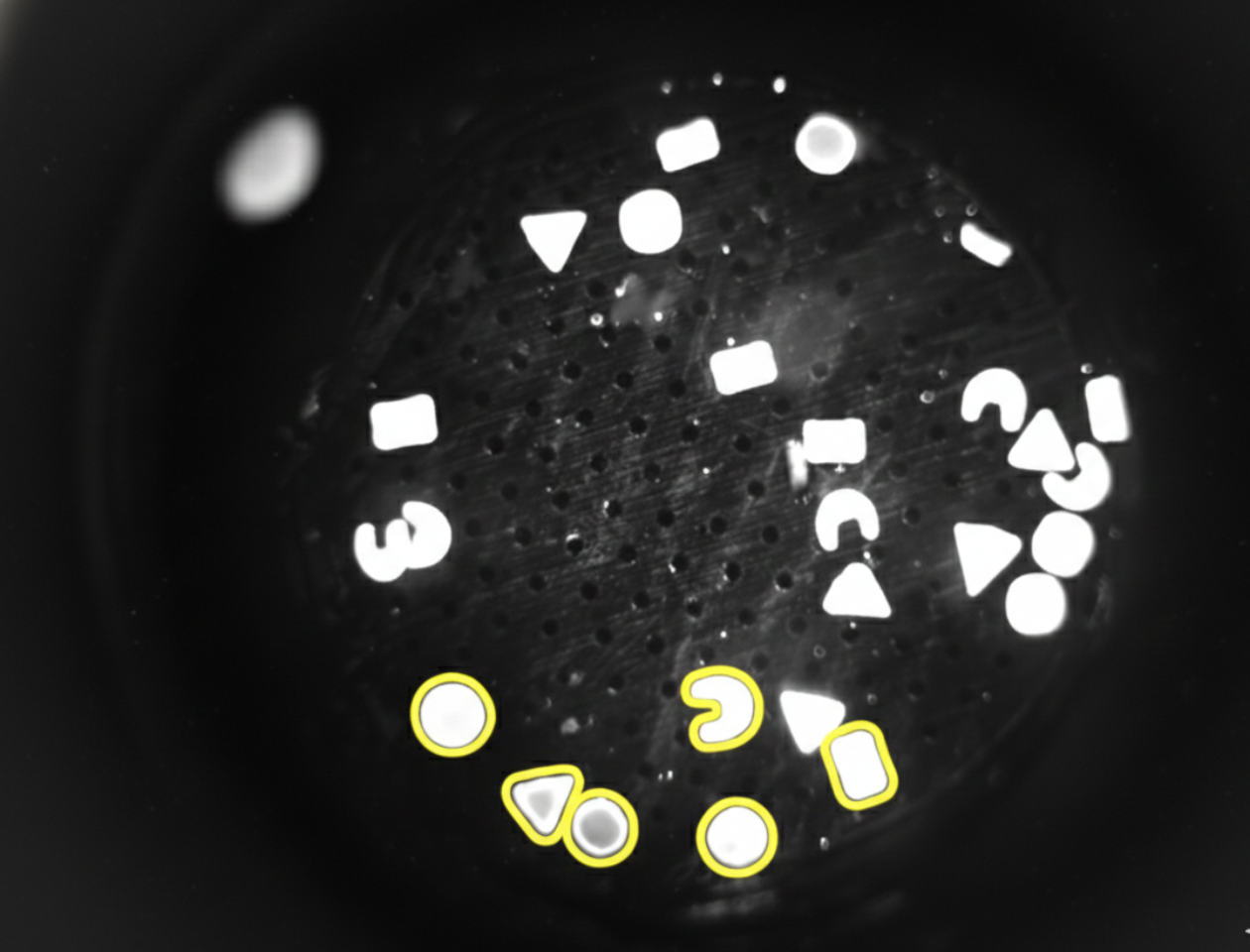
Multiplexed Analyte Detection
Technical Specifications

| Parameter | Specification |
|---|---|
| Dimensions (H/W/D) | 180 x 150 x 150 mm |
| Weight | 2.5 kg |
| Storage Conditions | 15–40°C |
| Power Supply Input | 12 V DC, 5 A, 60 W |
| Signal Detection | Fluorescence |

| Parameter | Specification |
|---|---|
| Dimensions | 74 × 40 × 15 mm |
| Weight | 18 g |
Compatible Sample Types
Whole Blood
(Finger prick/venous)
Serum
Nasal Swab
See our multiplexed test panels.
Our platform is adaptable
Have a novel POC assay? Let's build the next generation of diagnostics together.
Partnership Enquiry
Multiplexed, microfluidics based detection platform
High-sensitivity, quantitative results at the point of care.
Simple Workflow
Obtain sample
Prick the fingertip with a sterile lancet to collect the blood sample.
Add sample
Add the patient sample to the test cartridge.
Load cartridge
Place cartridge into the analyzer.
View Results
Run the test and view results within 35min.
See How It Works
The μFluidix Analyzer
Rapid Results
35 min
Multiplexed
Up to 5 analytes
Fully Automated
No manual intervention required
Easy User Interface
Minimal steps
The μFluidix Cartridge
Low Sample Volume
30 µL
Ready to Use
Pre-loaded with room temperature stable reagents
Smart Analyte Grouping
Targeted panels for the right combination of markers.
Compatible Sample Types
Whole Blood
Serum
Swab
Technical Specifications
| Parameter | Specification |
|---|---|
| Dimensions (H/W/D) | 180 x 150 x 150 mm |
| Weight | 2.5 kg |
| Storage Conditions | 15-40°C |
| Power Supply Input | 12 V DC, 5 A, 60 W |
| Signal Detection | Fluorescence |
| Parameter | Specification |
|---|---|
| Dimensions | 74mm x 40mm x 15mm |
| Weight | 18g |
| Storage | Placeholder |
Our platform is adaptable.
Have a novel POC assay? Let's build the next generation of diagnostics together.
See our multiplexed test panels.

QuantALFA - Lateral Flow Assay Reader
Transforming the standard rapid test into precision diagnostics.
Our Reader Technology
One reader for all tests

Rapid Scan
Readout of results within 5 seconds
High Accuracy
< 3% CV
Portable Design
Compact and pocket-size reader
Affordability
Cost-effective vs lab analyzers
Quantitative Output
Beyond typical LFA
Colorimetric Technology
High-contrast membrane chemistry
Streamlined Workflow

Obtain sample
Prick the fingertip with a sterile lancet to collect the blood sample.
Prepare the sample
Mix the sample with the provided buffer.
Add sample to the cassette
Add the buffer sample mix into the test cassette and incubate for 15 minutes.

Scan and view results
Scan the QR code using our QuantALFA app to view the results
See How It Works
QuantALFA App Ecosystem
Seamlessly pair the reader to the QuantALFA app to perform tests and log digital records.
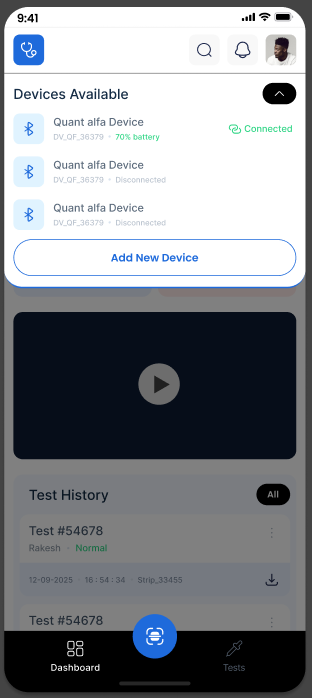
Easy Pairing
Connects wirelessly to the QuantALFA reader to sync test data.
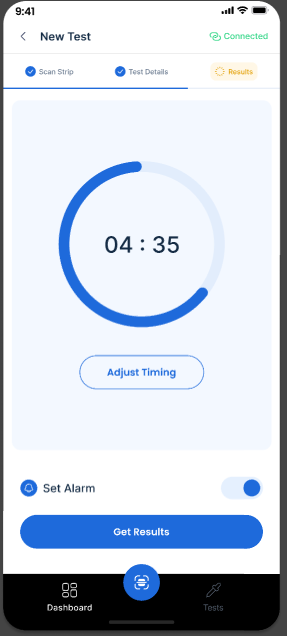
Guided Workflow
View detailed test information and step-by-step running instructions.
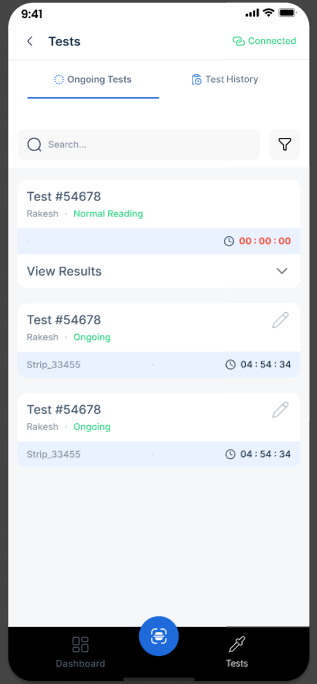
Accessible Records
Receive and store quantitative results directly on your mobile phone.
Our Available Tests
Renal Health
Early and accurate assessment of kidney function using sensitive with biomarker, Cystatin C.
Infection & Inflammation
Quantitative measurement of CRP to assess inflammation & infection severity.
Cardiac Care
High-sensitivity screening using hs-CRP for cardiovascular risk assessment.
μFluidix Diagnostic Panels
High-sensitivity, multiplexed panels for complex diagnostics.
Upcoming Test Panels
Fertility Panel
Assess reproductive health and support fertility treatments with a comprehensive hormonal profile from a single sample.
| Parameter | Specification |
|---|---|
| Analytes | FSH, LH, E2, P4 |
| Sample Type | Serum, Whole Blood |
| Time to Result | 35 minutes |
Thyroid Panel
Screen for, and monitor thyroid dysfunction on the spot with lab-quality quantitative results.
| Parameter | Specification |
|---|---|
| Analytes | TSH, Total T3, Total T4 |
| Sample Type | Serum, Whole Blood |
| Time to Result | 35 minutes |
Respiratory Panel
Quickly identify and distinguish between key respiratory pathogens to guide immediate, targeted treatment decisions.
| Parameter | Specification |
|---|---|
| Pathogens | Influenza A, Influenza B, RSV, COVID-19 |
| Sample Type | Nasal Swab |
| Time to Result | 35 minutes |
Panels in the Pipeline
Micronutrient Panel
Supported by the Gates Foundation
Anemia Panel
Supported by the Gates Foundation
Tropical Fever Panel
Development in Progress
Need high-sensitivity testing?
Request a Demo
.png)
QuantALFA™ Tests
Comprehensive testing solutions for qualitative and quantitative results.
Precision Beyond Binary Results
Quantitative Results
Fast & Accurate
CDSCO Approved
Available Test Kits
Cystatin-C
A sensitive biomarker for early and accurate assessment of kidney function.
| Parameter | Specification |
|---|---|
| Sample Type | Serum (S), Whole Blood (WB) |
| Sample Volume | 1 µL (S) or 2 µL (WB) |
| Detection Range | 0.5 - 10 µg/mL |
| Read time | 15 min |
| R2 | 0.95 |
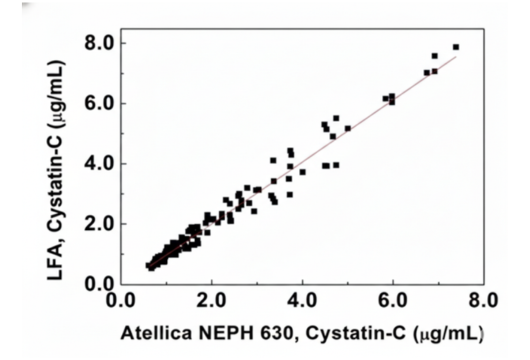
C-Reactive Protein (CRP)
Used for detection and evaluation of infection, tissue injury and inflammatory disorders.
| Parameter | Specification |
|---|---|
| Sample Type | Serum (S), Whole Blood (WB) |
| Sample Volume | 1 µL (S) or 2 µL (WB) |
| Detection Range | 2.5 - 200 µg/mL |
| Read time | 15 min |
| R2 | 0.96 |
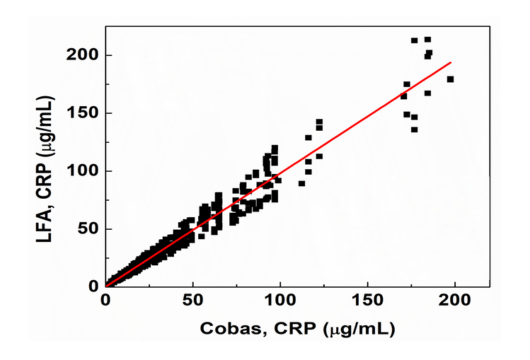
High-sensitivity C Reactive Protein (hs-CRP)
Helps predict heart disease, cardiovascular disease, and stroke risks.
| Parameter | Specification |
|---|---|
| Sample Type | Serum (S), Whole Blood (WB) |
| Sample Volume | 3 µL (S) or 5 µL (WB) |
| Read time | 15 min |
| Detection Range | 0.1 - 10 µg/mL |
| R2 | 0.97 |
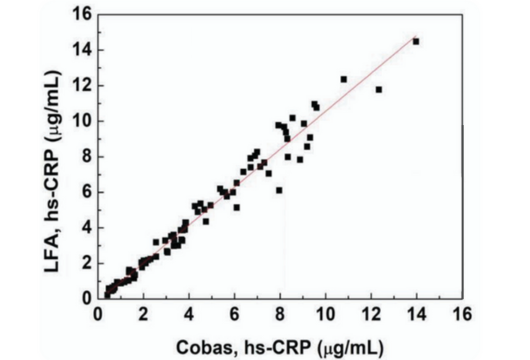
Tests in the Pipeline
HbA1c
Diabetes Management
Supported by Gates Foundation
Dengue NS1

Infectious Disease
TSH
Thyroid Function
PCT
Sepsis
Interested in our rapid tests?
Know more about our rapid tests

USelect: Superior Sperm Selection
The advanced solution for ART success.
Why USelect?
Traditional sperm selection methods like Swim-Up (SU) and Density Gradient (DG) focus solely on motility. USelect goes further by prioritizing sperm with optimal DNA integrity and acrosomal status, recreating the natural selection mechanism using Hyaluronic Acid (HA) binding.
Key Advantages
Superior Selection
Prioritizes optimal DNA integrity and acrosomal status.
Higher Success Rates in IUI
Achieved a 28% pregnancy rate per cycle vs 9-19% for traditional methods.
Lower DNA Fragmentation
Significantly reduces the DNA Fragmentation Index (DFI).
Efficient and Rapid
Sample processing takes less than 40 minutes.
Clinically Proven Results
3x Higher Success Rate
In a comparative IUI study, USelect achieved a 28% pregnancy rate per cycle.
-
✔
3x higher than Swim-Up
-
✔
~1.5x higher than Density Gradient
Implantation Rates Comparison
See How It Works
Clinical Data & Publications
Supporting Research
The performance and principles of the USelect technology are supported by extensive clinical research. For more data, please review the following publications.
Improve your ART success rates
Know more about USelect
Accelerating Diagnostic Innovation Through Partnership
We believe in the power of collaboration to solve global healthcare challenges. Whether you are looking for OEM services, assay development, or to partner with us as a distributor, Achira is your partner of choice.
Partnership Models
Technical Partnerships
Collaborate to co-develop new assays on our μFluidix platforms or broaden existing assay categories driven by clinical needs.
Commercial Partnerships
Facilitate market launch and distribution. We welcome partners with established commercial pathways to expand our product reach.
Our Capabilities
Assay Development
Biochemistry, Immunology, and Molecular Biology Expertise.
Design & Prototyping
Rapid translation of ideas into manufacturable products.
Manufacturing
ISO 13485 compliant facility for microfluidic devices.
Our platform is adaptable
Have a novel POC assay? Let's build the next generation of diagnostics together.
Partnership Enquiry
About Achira Labs
Pioneering frictionless, point-of-care testing to bridge the gap between clinical labs and patient needs.
Our Story
Founded in 2009 in Bengaluru, India, Achira Labs began with a vision to decentralize medical diagnostics. We recognized that while central labs offer precision, they often lack speed and accessibility for patients in need of immediate care.
By leveraging proprietary microfluidics and hydrogel technology, we have developed platforms that shrink the lab onto a chip. Today, we are an ISO 13485 compliant company, transforming how diagnostics are delivered across India and beyond.
Our Mission
To empower patients and doctors with convenient, timely access to affordable and accurate medical testing.
Accessibility
We believe geography should not dictate health outcomes. Our portable platforms bring sophisticated testing to clinics, small labs, and remote settings.
Accuracy
We do not compromise on quality for speed. Our microfluidic technology ensures lab-grade precision in a rapid format.
Empowerment
By providing immediate quantitative results, we empower clinicians to make informed treatment decisions on the spot, reducing anxiety and improving care.
Our Vision
A world where advanced medical diagnostics are not a luxury, but a standard of care available to everyone. We aim to be a global leader in point-of-care testing by continuously innovating at the intersection of biology, engineering, and connected health.
Investors & Funders
We are proud to collaborate with leading organizations to advance healthcare access.
Partners & Collaborators
Collaborate with our R&D team.
Intellectual Property & Publications
Pioneering research backed by a robust portfolio of patents and peer-reviewed studies.
Granted Patents
| Patent Number | Title |
|---|---|
| US 8,647,742 | Diagnostic gel composition, method for making a diagnostic gel composition |
| US 9,714,912 B2 | Compositions for fabric based lateral flow assay device using electrochemical detection means |
| CN 102811815 B | Method for making and using a diagnostic element |
| JP 5661795 B2 | Diagnostic Element, and a diagnostic device comprising a diagnostic element |
| US 2018/0072987 A1 | Method for extracting viable sperms from a seme.n sample (USelect Technology) |
| JP 5775157 B2 | Methods of making a diagnostic device by interweaving hydrophobic and hydrophilic fibers |
Key Scientific Publications
The Quantitative Detection of Cystatin-C in Patient Samples Using a Colorimetric Lateral Flow Immunoassay
Santosh Kumar Bikkarolla et al. (2024)
A collaborative effort with Vision Research Foundation Chennai, validating our QuantALFA platform for renal function testing.
A hydrogel sensor-based microfluidic platform for the quantitative and multiplexed detection of fertility markers
Anal Methods, 2019, 11, 1639-1650
Describes the core technology behind our μFluidix Fertility Panel, demonstrating high sensitivity for point-of-care immunoassays.
Woven electrochemical fabric-based test sensors (WEFTS): a new class of multiplexed electrochemical sensors
Lab on a Chip, 2015, 15, 2064-2072
Explores our innovative "Lab-on-Fabric" technology for creating low-cost, multiplexed sensors.
Wearable Woven Electrochemical Biosensor Patch for Non‐invasive Diagnostics
Electroanalysis, 2016, Volume 28, Issue 6
Research into non-invasive diagnostic solutions using wearable textile sensors.
'Fab-chips': a versatile, fabric-based platform for low-cost, rapid and multiplexed diagnostics
Lab on a Chip, 2011, 11, 2493-2499
Foundational paper establishing the viability of fabric-based microfluidics for rapid diagnostics.
Fabric Technology
Achira has developed a revolutionary new fabric-based technology to perform lateral flow and electrochemical assays. Using our patented approach, yarn coated with reagents is woven into fabric that can be cut into strips to make individual or multiplexed test sensors.
The inherent scalability of the textile manufacturing process leads to significant cost savings. This platform lends itself easily to low-resource settings, with the potential to use local skills and networks for assembly.
Electrochemical Sensors
Yarns pre-coated with conducting inks and reagents are woven to mass-manufacture sensors in a single step, offering a lower-cost alternative to screen printing.
Immunosensors
Lateral-flow immunosensors built on fabric. The entire assembly—conjugate reagents, test lines, and control lines—is assembled in one step on a loom.
Weaving Health & Livelihood
"Achira has partnered with community organizations to weave and assemble diagnostic strips at the point of care, generating employment and increasing local availability of tests."
Want to learn more about our work?
Board of Directors

Dr. Dhananjaya Dendukuri
CEO & Managing Director

Suri Venkatchalam
Director

Achin Gupta
Director
Advisors

Patrick Doyle
Scientific Advisor

Dr Ashutosh Mundkur
Advisor

Dr Lalathaksha M Kumbar
Advisor

Dr Sujay Prasad
Advisor
Our Team

Dr. Dhananjaya Dendukuri
CEO & Co-Founder
—
—

Dr. Vinayaka Aaydha
Head – R&D and Platform Integration
Dr. Vinayaka Aaydha on LinkedIn
Dr. Vinayaka leads the Research & Development and Platform Integration divisions at Achira Labs, bringing over 18 years of pioneering research experience to the organization’s leadership team. Since January 2024, he has directed the innovation of advanced analytical methods, overseeing the seamless convergence of molecular biology and immunoassays—including ELISA, CLIA, and FLIA within stringent Quality Management Systems (QMS).
A globally recognized authority in Point-of-Care (POC) diagnostics, Dr. Vinayaka specializes in the architecture of next-generation diagnostic platforms. His work is defined by a multidisciplinary approach, integrating complex bioanalytical assays with high-fidelity optical detection modules to deliver clinical-grade accuracy in portable formats.
Core Competencies & Strategic Impact
- Platform Innovation: Expert in transferring sophisticated bioanalytical assays to proprietary POC platforms, ensuring rigorous validation and performance stability.
- Advanced Biosensor Design: Specialized in the development of multiplexed biosensors and the integration of optical detection systems, including fluorescence, luminescence, turbidimetry, and absorbance.
- Leadership & Collaboration: A proven track record in building high-performance scientific teams and fostering cross-functional synergy to drive end-to-end product innovation.
Distinguished Fellowships & Academic Excellence
Dr. Vinayaka’s career is underpinned by prestigious international recognition, reflecting his contribution to the global scientific community. His accolades include:
- Marie Curie Fellowship (Technical University of Denmark)
- CSIR-Nehru Postdoctoral Fellowship (IMTECH, Chandigarh)
- Senior Research Fellowship (CFTRI, Mysore)
His leadership ensures that Achira Labs continues to push the boundaries of diagnostic technology, translating complex laboratory science into robust, real-world medical solutions.

Komal Bhanushali
Head - LFA
Komal Bhanushali on LinkedIn
Komal is a results-driven R&D leader with over nine years of specialized expertise in the design, development, and commercialization of advanced In-Vitro Diagnostics (IVD). At Achira Labs, she spearheads the innovation of high-performance Lateral Flow Assays (LFAs), transforming rapid testing into a tool of quantitative precision for global healthcare markets.
Throughout her career, Komal has been at the forefront of diagnostic evolution, moving beyond qualitative "yes/no" results to deliver sophisticated, high-sensitivity diagnostic platforms. Her work spans a broad spectrum of clinical applications, including critical biomarkers for acute kidney injury (NGAL) and systemic inflammation (CRP), as well as high-impact infectious diseases such as Malaria, HIV, and COVID-19.
Technological Breakthroughs & Innovation
- Nanotechnology Advancement: Developed proprietary gold nanoshell particles that achieved a revolutionary 100-fold increase in assay sensitivity, pushing the boundaries of detection limits for rapid diagnostics.
- Digital Health Integration: Co-inventor of a patented, smartphone-based reader technology that enables quantitative, lab-grade results on standard LFA platforms—an essential step in the democratization of precision diagnostics.
- Full-Cycle Product Development: Leads the end-to-end journey from foundational molecular design to large-scale commercialization, ensuring that innovative concepts are translated into reliable, market-ready diagnostic tools.
- Clinical Impact: Dedicated to the development of assays that provide immediate, actionable data, significantly reducing the time-to-treatment in resource-limited and point-of-care settings.
Komal’s technical expertise in biochemistry and nanotechnology, combined with her focus on digital connectivity, ensures that Achira Labs remains a leader in the next generation of smart, high-sensitivity rapid diagnostics.

Dr. Bhavna Goyal
Head - Clinical Studies
Bhavna Goyal on LinkedIn
Dr. Bhavna Goyal leads the Clinical Studies division at Achira Labs, where she oversees the rigorous validation of diagnostic platforms and medical devices. With over 15 years of research experience—including nearly a decade dedicated to the diagnostics industry—Dr. Goyal is the primary architect of Achira’s clinical evidence strategies, ensuring that every product meets the highest benchmarks of accuracy, reliability, and global regulatory compliance.
A scientist of international standing, Dr. Goyal holds a Ph.D. in Life Sciences from the International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi. She is responsible for the vital transition of diagnostic innovations from the laboratory to real-world clinical application.
Clinical Leadership & Strategic Alliances
- Validation & Regulatory Governance: Directs the end-to-end clinical validation process, ensuring that diagnostic performance meets stringent regulatory mandates and clinical efficacy standards.
- Institutional Partnerships: Manages strategic collaborations with NABL-accredited laboratories and premier hospitals, overseeing the legal and ethical frameworks of MOUs, NDAs, and Institutional Ethics Committee (IEC) approvals.
- Clinical Sample Stewardship: Leads the acquisition of clinical biosamples and manages feasibility assessments to support internal validation and pilot studies.
- Multiplex Assay Innovation: Possesses deep technical expertise in developing multiplexed assays for hormone panels, infectious diseases, and renal biomarkers.
- Evidence-Based Excellence: Drives a culture of scientific rigor, supported by her extensive background in advanced data analysis, biochemistry, and molecular biology, as evidenced by her portfolio of peer-reviewed publications and international grants.
Dr. Goyal’s commitment to scientific integrity ensures that Achira Labs’ solutions are not only innovative but are clinically proven tools ready to enhance patient outcomes in diverse healthcare settings worldwide.

Kunal Singh
Head - Manufacturing
Kunal Singh on LinkedIn
Kunal Singh is the Head of Manufacturing at Achira Labs, where he oversees the transition of advanced diagnostic innovations into high-volume, precision-engineered medical devices. A Mechanical Engineer (B.Tech, MDU) with over a decade of cross-industry experience in the medical device and automotive sectors, Kunal specializes in the application of core industrial engineering principles to sophisticated healthcare manufacturing.
At Achira, he is responsible for the strategic orchestration of production planning, facility management, and the integration of robust Quality Management Systems (QMS). His leadership ensures that the manufacturing floor operates with the agility and precision required to meet global demand while maintaining uncompromising product integrity.
Manufacturing Excellence & Strategic Impact
- Process Engineering: Specializes in structured problem-solving and advanced process studies, including Value Stream Mapping (VSM), line balancing, and the implementation of standardized work protocols.
- Scalable Production: Leverages his deep background in high-precision automotive manufacturing to implement "zero-defect" production strategies within the medical device ecosystem.
- Manpower & Resource Management: Adept at leading multidisciplinary manufacturing teams, fostering a culture of safety, efficiency, and collective accountability.
- Data-Driven Performance: As a Lean Six Sigma Black Belt, Kunal utilizes rigorous data analytics to refine manufacturing workflows, minimize waste, and ensure the cost-effective delivery of high-quality diagnostics.
Kunal’s commitment to manufacturing excellence ensures that Achira Labs consistently delivers reliable, high-fidelity diagnostic tools to clinicians and patients worldwide, bridging the gap between innovative design and large-scale clinical utility.

Dr. Christy Rosaline
Manager - Biomanufacturing & Process Development
Dr. Christy Rosaline on LinkedIn
Dr. S. Christy Rosaline is a distinguished manager at Achira Labs, where she spearheads the development and clinical validation of next-generation diagnostic solutions. With a focus on high-acuity needs—including rapid UTI detection, advanced antibiotic susceptibility testing (AST), and precision lateral flow assays—Dr. Rosaline ensures that scientific innovation meets the rigorous demands of modern medicine.
Combining deep technical acumen with a commitment to quality, she leads the end-to-end lifecycle of diagnostic R&D, from initial product design to stringent regulatory compliance and pilot launch. Her work is central to Achira’s mission of delivering diagnostic accuracy at the point of care.
Areas of Expertise
- Translational R&D: Over six years of experience in microbiology, molecular biology, and biosensor technology, successfully moving products from benchtop concepts to clinical applications.
- Clinical Leadership: Expert oversight of multi-platform clinical trials for infectious diseases, ensuring robust data integrity and alignment with global healthcare standards.
- Regulatory & Quality Excellence: Dedicated focus on quality control (QC) frameworks and regulatory pathways to ensure patient safety and product reliability.
- Academic Distinction: A Ph.D. in Biotechnology and a recipient of multiple prestigious ICMR (Indian Council of Medical Research) fellowships, underscoring her contributions to the scientific community.

Dr. Vikas Ghattargi
Manager - Quality Assurance, Regulatory Affairs
Dr. Vikas Ghattargi on LinkedIn
Dr. Vikas is a distinguished specialist in Quality Assurance and Regulatory Affairs, dedicated to establishing the high-integrity compliance frameworks that anchor Achira Labs diagnostic innovations. With a focus on the intersection of scientific excellence and global regulatory mandates, he ensures that every solution in the Achira portfolio adheres to the most stringent international benchmarks for patient safety and clinical efficacy.
A Certified Lead Auditor for ISO 13485 and ISO 9001, Dr. Vikas architected the organization’s robust Quality Management Systems (QMS). His leadership is pivotal in navigating the complex pathways of regulatory submissions, ensuring that Achira’s breakthroughs transition seamlessly from the laboratory to the global medical market.
Core Expertise & Strategic Governance
- Regulatory Lifecycle Management: Orchestrates comprehensive regulatory strategies and submissions, ensuring continuous alignment with evolving global healthcare policies.
- Risk & Standard Governance: Advanced expertise in ISO 14971 (Risk Management) and ASTM standards, applying clinical-grade rigor to identify and mitigate risks throughout the product lifecycle.
- Specialized Systems: Deep technical proficiency in Software as a Medical Device (SaMD) and Software in a Medical Device (SiMD), critical for the next generation of interconnected diagnostic platforms.
- Operational Precision: As a Lean Six Sigma Black Belt, he integrates data-centric methodologies to optimize manufacturing processes and drive continuous organizational improvement.
- Quality Advocacy: Leads cross-functional teams to foster a culture of uncompromising quality, reinforcing the trust that clinicians and stakeholders place in Achira Labs.
Through his commitment to exceeding international standards, Dr. Vikas ensures that Achira Labs remains a benchmark for reliability and excellence in the diagnostic industry, supporting the company’s mission to deliver superior patient outcomes.

Siddarth R
Head - Program Management
Siddharth R on LinkedIn
Sid serves as the Head of Program Management at Achira Labs, where he orchestrates the complex lifecycle of medical device development from initial concept to global market delivery. With an engineering foundation and over seven years of specialized experience in high-stakes instrumentation and diagnostics, he acts as the strategic nexus between R&D, Engineering, Operations, and Quality.
Sid is responsible for driving organizational momentum, ensuring that Achira’s ambitious technical roadmap is met with disciplined execution and rigorous governance. He specializes in creating the transparent workflows and robust data-integrity frameworks necessary to navigate the highly regulated diagnostic landscape with both agility and precision.
Strategic Oversight & Program Governance
- Cross-Functional Integration: Acts as the primary bridge between multidisciplinary teams, harmonizing scientific breakthroughs with engineering realities and operational scale.
- Regulatory-Aligned Development: Expertly navigates the integration of quality systems and regulatory requirements within the development pipeline, ensuring that speed-to-market never compromises compliance.
- Operational Excellence: Passionate about the design of smarter governance systems that foster a culture of accountability, decision-making clarity, and sustained momentum.
- Instrumentation & Technical Expertise: Leverages a strong engineering background to oversee the technical requirements of diagnostic hardware, ensuring alignment with both clinical needs and manufacturing capabilities.
- Data Integrity & Risk Mitigation: Focuses on strengthening internal data frameworks to support audit readiness and reliable product performance, empowering teams to execute with confidence.
By establishing the frameworks that allow innovation to scale, Sid ensures that Achira Labs remains a reliable and efficient partner to healthcare providers and global distributors, delivering life-impacting diagnostic solutions with world-class precision.

Faisal Ahmed
Manager - Business Development
Faisal Ahmed on LinkedIn
Faisal is a seasoned leader with over nine years of cross-functional experience driving growth at the intersection of pharmaceutical innovation and medical diagnostics. As a key strategist at Achira Labs, he orchestrates the alignment of commercial objectives with scientific breakthroughs, overseeing the full product lifecycle—from foundational R&D to global market entry.
Throughout his career, Faisal has demonstrated a consistent ability to scale complex diagnostic portfolios and optimize business performance. His professional highlights include:
- Portfolio Excellence: Successfully spearheaded the launch of over 350 high-impact products across diverse therapeutic segments.
- Strategic Outreach: Established a premier "scientist-to-scientist" engagement framework, fostering deep institutional collaborations and technical synergy.
- Operational Leadership: Managed critical large-scale initiatives, including high-volume COVID-19 diagnostic operations and multidisciplinary team leadership.
- Technical Innovation: Driven advancements in recombinant antibody technologies and pioneered CRISPR-based validation protocols, resulting in significant intellectual property contributions and patent filings.
Faisal’s expertise ensures that Achira Labs remains at the forefront of the diagnostic industry, delivering precision-engineered solutions that address the evolving needs of global healthcare.

Raghavendra Katti
General Manager – Operations
Raghavendra Katti on LinkedIn
Raghavendra has been with Achira Labs since 2015, bringing a robust 18-year tenure in corporate finance, human capital management, and strategic operations. With a Postgraduate Diploma in Business Management (Finance) and a foundational career built within high-stakes export environments, he provides the fiscal and operational stewardship necessary to scale a leading-edge medical technology firm.
As a core member of the leadership team, Raghavendra is responsible for the architectural integrity of Achira’s business processes. He ensures that the company’s ambitious scientific goals are matched by rigorous financial planning and operational efficiency, facilitating a seamless bridge between innovation and market execution.
Strategic & Operational Leadership
- Financial Stewardship: Expertly manages accounts and financial strategy, optimizing resource allocation to support sustained long-term growth and organizational stability.
- Operational Optimization: Streamlines complex internal workflows and supply chain operations, leveraging his extensive background in export-house logistics to enhance corporate agility.
- Corporate Governance: Drives excellence across key business functions, maintaining the disciplined infrastructure required to navigate the dynamic and highly regulated medical diagnostic industry.
Raghavendra’s pragmatic leadership and dedication to operational effectiveness are fundamental to Achira Labs’ ability to deliver life-impactful technologies consistently and at scale. His commitment to cross-functional synergy empowers the organization to thrive in an increasingly complex global healthcare landscape.
What Our Partners Say
Trusted by clinicians and industry leaders worldwide.
Industry Recognition
"Our decision to invest more in Achira is backed by Cipla's commitment to advance in the PoC diagnostics space. With the aim to reduce the existing gap in the ecosystem, our strategic financing will enable Achira to commercially launch and further develop test panels."
Mr. Achin Gupta
CEO – One India Business, Cipla Limited
"We are proud to invest in these solutions and celebrate their remarkable impact. Innovations like these are showing how technology can improve care for millions of women, ensuring safer births and healthier beginnings."
Dr. Karlee Silver
CEO, Grand Challenges Canada
From the Clinic
[Doctor Name]
IVF Specialist, Mumbai
"Using USelect has significantly improved our IUI success rates. The workflow is simpler and faster than our previous density gradient method."
[Lab Director Name]
Diagnostic Center, Bangalore
"The QuantALFA reader is incredibly easy to use. We can now offer quantitative CRP results in minutes, which our patients appreciate."
[Clinic Name]
Rural Health Clinic
"Achira's platform allows us to perform critical thyroid tests without sending samples away. It has transformed how we treat our patients."
Join our growing network of partners.
Grants & Recognitions
A history of innovation supported by global leaders.
2025
C-Camp
Product Development GrantAwarded a grant under the C-CAMP One Health AMR Challenge, supported by ICARS to develop and validate a rapid point-of-care test to distinguish bacterial from viral Infections in humans
2024
Gates Foundation
Product Development GrantAwarded a grant to support the development of our Anemia Panel and HbA1c test. Focused on improving maternal and child health outcomes in low-resource settings.
2016
National Award
Indigenous Product Commercialization2013

BIRAC Innovator's Award
Best Pitch2013

Grand Challenges Canada
Seed GrantJoin us in our journey of innovation.
Blog & News
Insights on technology, clinical impact, and company updates.
The Science Behind Our 3D Hydrogels
Lorem ipsum dolor sit amet, consectetur adipiscing elit...
Read More »Cystatin C vs. Creatinine: The POCT Impact
Ut enim ad minim veniam, quis nostrud exercitation ullamco...
Read More »Beyond Motility: Why DNA Fragmentation Matters
Duis aute irure dolor in reprehenderit in voluptate velit esse...
Read More »Achira Labs Receives CDSCO Approval
Excepteur sint occaecat cupidatat non proident, sunt in culpa...
Read More »Quantifying LFA: Our Calorimetric Method
Lorem ipsum dolor sit amet, consectetur adipiscing elit...
Read More »Want to learn more?
Product Documentation
Access brochures, manuals, and safety data sheets for our products.
QuantALFA Test Kits
Cystatin-C
Renal Function Test
CRP
C-Reactive Protein
hs-CRP
High-Sensitivity CRP
μFluidix Panels
Fertility Panel
FSH, LH, E2, P4
Thyroid Panel
TSH, T3, T4
Respiratory Panel
Flu A/B, RSV, Covid-19
Sperm Selection
USelect Kit
Magnetic Bead Separation
Build the Future of Diagnostics.
Join a multidisciplinary team of engineers, scientists, and visionaries working to make healthcare accessible to everyone, everywhere.
Why Join Achira?
Innovation First
Work at the cutting edge of microfluidics and hydrogel chemistry. We encourage out-of-the-box thinking to solve complex biological problems.
Impact
Your work directly contributes to saving lives. From rural clinics to high-tech labs, our products bridge critical gaps in healthcare delivery.
Our Culture
We believe in a flat hierarchy where ideas triumph over titles. Collaborate freely across R&D, manufacturing, and business teams.
Life at Achira











Current Openings
QA Assistant +
Bengaluru • Full-time • Operations
We are looking for a QA Assistant to support quality assurance activities across manufacturing and testing operations. The role involves documentation, SOP compliance, audits, and coordination with cross-functional teams.
- Support QA documentation and reporting
- Assist in internal and external audits
- Ensure compliance with regulatory standards
Don’t see a role that fits? We are always looking for exceptional talent.
Submit Your Profile
Contact Us
FIND US HERE
Contact us by email: services@achiralabs.com
Visit us @ our office: 66b, 13th Cross Rd, Dollar Layout, Bengaluru – 560078, India
Call our Support team: +91 – 97407 22549
Fill out the form below and a member of our team will get back to you shortly.


















